|
Foodlaw-Reading
Dr David Jukes, The University of
Reading, UK
Providng access to food law since May 1996 |
 |
.....  ..... ..... ..... .....  ..... .....  ..... .....  ..... ..... 
|
 Please complete a quick survey on a Google Form - LINK
Please complete a quick survey on a Google Form - LINK
Last updated:
27 March, 2024
Improvement Agents: Food Additives
Providing access to EU and UK legislation
On this page:
- Summary - Brief details of the chronology of developments linked to this topic
- EU Legislation - description of the legislation controlling additives
in the EU with links to EU documents
- UK Legislation - links to the UK legislation
|
Summary
The adoption of a single harmonised list of food additives for the European Union has taken a long time. It can be split into 3 separate stages:
- Stage 1: Early EU legislation on food additives focused on the need to create single lists for each functional group of additives. The first directive to be agreed was for colours (1962) and used the E-number classification system for the first time. This was followed by directives for preservatives (1964), antioxidants (1970) and emulsifiers, stabilisers, thickeners and gelling agents (1974). Adoption of these was slow and the resulting directives only specified the list of permitted substances. Member States were still free to specify which foods could contain the substances and the maximum permitted levels.
- Stage 2: As part of the moves to create the Internal Market, the Community agreed that the control of food additives should be fully harmonised throughout the Community. The earlier attempts (Stage 1) had only been partially successful and for the purposes of the Internal Market, full agreement was needed. This was achieved during the period 1988-1995 and was based on a framework contained in Directive 89/107 with the more detailed controls contained in separate directives - 3 specific directives covering (1) colours, (2) sweeteners and (3) all remaining additives, and supporting directives containing purity criteria. This was much more successful but retained a lengthy decision-making process and, by using directives, required national implementation.
- Stage 3: In 2008, a new broader framework was agreed based on a Regulation which also incorporates flavourings and enzymes - commonly grouped together as 'Food Improvement Agents'. Specific controls for additives were applied by the new Regulation 1333/2008. This entered into force on 20 January 2010. During a transitional period, the detailed technical provisions of the supporting directives remained in force. However in November 2011 the detailed provisions designed to replace the requirements of the directives were published in Regulation 1129/2011, which introduced substantial amendments to Regulation 1333/2008, and these entered into force on 1 June 2013. Updated purity criteria were published in Regulation 231/2012.
The following figure illustrates how the controls of food additives are part of the overall control of food improvement agents:
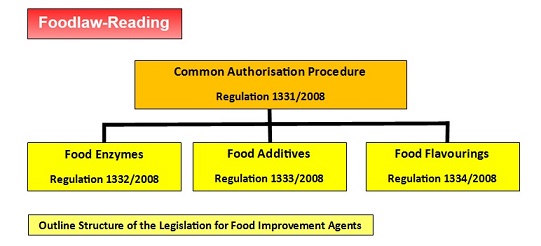
Note that for EU legislative purposes, food flavourings are not considered as food additives and are covered by separate legislation based on Regulation 1334/2008 [For details, see the separate page: Food Flavourings].
Some aspects of the chronology of the control of food additives (Stages 2 and 3) are illustrated in this figure:
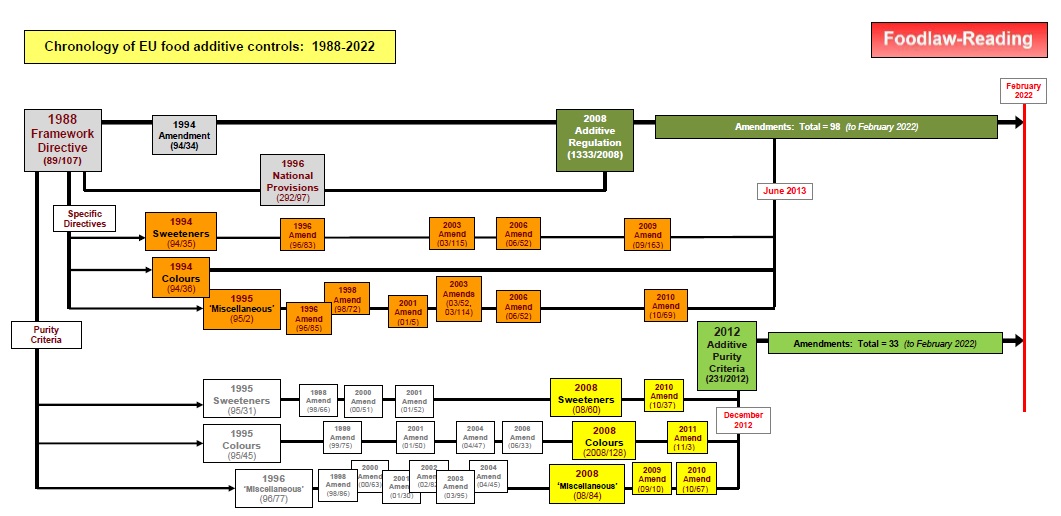
For a larger version of this figure as a pdf file, Click Here
For the Commission's page on this topic, see: Food Improvement Agents.
EU Legislation
Stage 3 - Controls based on Regulation 1333/2008
The process for authorising food additives, food enzymes and food flavourings is provided by a 'common authorisation procedure' regulation - Regulation 1331/2008 (as amended) :
- Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings (OJ L354, 31.12.2008, page 1)
Amendments:
- Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC (OJ L231, 6.9.2019, page 1)
The general and the detailed controls on food additives are now contained in a single document - Regulation 1333/2008 (as amended) :
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (OJ L354, 31.12.2008, page 16)
Amendments:
- Commission Regulation (EU) No 238/2010 of 22 March 2010 amending Annex V to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with regard to the labelling requirement for beverages with more than 1,2 % by volume of alcohol and containing certain food colours (OJ L75, 23.3.2010, page 17) [Minor amendment relating to alcoholic drinks]
- Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (OJ L295, 12.11.2011, page 1)
[Full listing of permitted additives, permitted foods and maximum levels for all additives]
- Commission Regulation (EU) No 1130/2011 of 11 November 2011 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives by establishing a Union list of food additives approved for use in food additives, food enzymes, food flavourings and nutrients (OJ L295, 12.11.2011, page 178)
[Separate listing of additives permitted for use in other additives, etc]
- Commission Regulation (EU) No 1131/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with regard to steviol glycosides (OJ L295, 12.11.2011, page 205) [Authorising the use of a new sweetener in certain specified foods]
- Commission Regulation (EU) No 232/2012 of 16 March 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the conditions of use and the use levels for Quinoline Yellow (E 104), Sunset Yellow FCF/Orange Yellow S (E 110) and Ponceau 4R, Cochineal Red A (E 124) (OJ L78, 17.3.2012, page 1) [Reducing the permitted levels and foods for these colours so as to reduce consumption levels]
- Commission Regulation (EU) No 380/2012 of 3 May 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the conditions of use and the use levels for aluminium-containing food additives (OJ L119, 4.5.2012, page 14) [Reducing the number of permitted aluminium additives as consumption levels of aluminium are considered to be too high]
- Commission Regulation (EU) No 570/2012 of 28 June 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of benzoic acid — benzoates (E 210-213) in alcohol-free counterparts of wine (OJ L169, 29.6.2012, page 43) [Permitting use of benzoates in alcohol-free wine]
- Commission Regulation (EU) No 583/2012 of 2 July 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polysorbates (E 432-436) in coconut milk (OJ L173, 3.7.2012, page 8) [Permitting use of polysorbates in coconut milk]
- Commission Regulation (EU) No 675/2012 of 23 July 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Talc (E 553b) and Carnauba wax (E 903) on unpeeled coloured boiled eggs and the use of Shellac (E 904) on unpeeled boiled eggs (OJ L196, 24.7.2012, page 52) [Minor technical amendments]
- Commission Regulation (EU) No 1049/2012 of 8 November 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyglycitol syrup in several food categories (OJ L310, 9.11.2012, page 41)
- Commission Regulation (EU) No 1057/2012 of 12 November 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of dimethyl polysiloxane (E 900) as an anti-foaming agent in food supplements (OJ L313, 13.11.2012, page 11)
- Commission Regulation (EU) No 1147/2012 of 4 December 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of beeswax (E 901), carnauba wax (E 903), shellac (E 904) and microcrystalline wax (E 905) on certain fruits (OJ L333, 5.12.2012, page 34)
- Commission Regulation (EU) No 1148/2012 of 4 December 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sulphur dioxide — sulphites (E 220-228) and propane-1, 2-diol alginate (E 405) in fermented grape must-based drinks (OJ L333, 5.12.2012, page 37)
- Commission Regulation (EU) No 1149/2012 of 4 December 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of extracts of rosemary (E 392) in fillings of stuffed dry pasta (OJ L333, 5.12.2012, page 40)
- Commission Regulation (EU) No 1166/2012 of 7 December 2012 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of dimethyl dicarbonate (E 242) in certain alcoholic drinks (OJ L336, 8.12.2012, page 75)
- Commission Regulation (EU) No 25/2013 of 16 January 2013 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the food additive potassium diacetate (OJ L13, 17.1.2013, page 1)
- Commission Regulation (EU) No 244/2013 of 19 March 2013 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Tricalcium phosphate (E 341 (iii)) in nutrient preparations intended for use in foods for infants and young children (OJ L77, 20.3.2013, page 3)
- Commission Regulation (EU) No 256/2013 of 20 March 2013 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Sodium ascorbate (E 301) in vitamin D preparations intended for use in foods for infants and young children (OJ L79, 21.3.2013, page 24)
- Commission Regulation (EU) No 438/2013 of 13 May 2013 amending and correcting Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of certain food additives (OJ L129, 14.5.2013, page 28)
- Commission Regulation (EU) No 509/2013 of 3 June 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of several additives in certain alcoholic beverages (OJ L150, 4.6.2013, page 13)
- Commission Regulation (EU) No 510/2013 of 3 June 2013 amending Annexes I, II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of iron oxides and hydroxides (E 172), hydroxypropyl methyl cellulose (E 464) and polysorbates (E 432-436) for marking of certain fruits (OJ L150, 4.6.2013, page 17)
- Commission Regulation (EU) No 723/2013 of 26 July 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of extracts of rosemary (E 392) in certain low fat meat and fish products (OJ L202, 27.7.2013, page 8)
- Commission Regulation (EU) No 738/2013 of 30 July 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of certain additives in seaweed based fish roe analogues (OJ L204, 31.7.2013, page 32)
- Commission Regulation (EU) No 739/2013 of 30 July 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Stigmasterol-rich plant sterols as a stabiliser in ready-to-freeze alcoholic cocktails, and the Annex to Commission Regulation (EU) No 231/2012 as regards specifications for Stigmasterol-rich plant sterols food additive (OJ L204, 31.7.2013, page 35)
- Commission Regulation (EU) No 816/2013 of 28 August 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Neutral methacrylate copolymer and Anionic methacrylate copolymer in solid food supplements and the Annex to Commission Regulation (EU) No 231/2012 as regards the specifications for Basic methacrylate copolymer (E 1205), Neutral methacrylate copolymer and Anionic methacrylate copolymer (OJ L230, 29.8.2013, page 1)
- Commission Regulation (EU) No 817/2013 of 28 August 2013 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Octenyl succinic acid modified gum arabic (OJ L230, 29.8.2013, page 7)
- Commission Regulation (EU) No 818/2013 of 28 August 2013 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Sucrose esters of fatty acids (E 473) in flavourings for water based clear flavoured drinks (OJ L230, 29.8.2013, page 12)
- Commission Regulation (EU) No 913/2013 of 23 September 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sweeteners in certain fruit or vegetable spreads (OJ L252, 24.9.2013, page 11)
- Commission Regulation (EU) No 1068/2013 of 30 October 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of diphosphates (E 450), triphosphates (E 451) and polyphosphates (E 452) in wet salted fish (OJ L289, 31.10.2013, page 58)
- Commission Regulation (EU) No 1069/2013 of 30 October 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sodium phosphates (E 339) in natural casings for sausages (OJ L289, 31.10.2013, page 61)
- Commission Regulation (EU) No 1274/2013 of 6 December 2013 amending and correcting Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards certain food additives (OJ L328, 7.12.2013, page 79)
- Commission Regulation (EU) No 59/2014 of 23 January 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sulphur dioxide — sulphites (E 220-228) in aromatised wine-based products (OJ L21, 24.1.2014, page 9)
- Commission Regulation (EU) No 264/2014 of 14 March 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyvinylpyrrolidone-vinyl acetate copolymer in solid food supplements and the Annex to Commission Regulation (EU) No 231/2012 as regards its specifications (OJ L76, 15.3.2014, page 22)
- Commission Regulation (EU) No 298/2014 of 21 March 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Magnesium dihydrogen diphosphate for use as raising agent and acidity regulator (OJ L89, 25.3.2014, page 36)
- Commission Regulation (EU) No 497/2014 of 14 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Advantame as a sweetener (OJ L143, 15.5.2014, page 6)
- Commission Regulation (EU) No 505/2014 of 15 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of caramel colours (E 150a-d) in beer and malt beverages (OJ L145, 16.5.2014, page 32)
- Commission Regulation (EU) No 506/2014 of 15 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Ethyl lauroyl arginate as a preservative in certain heat-treated meat products (OJ L145, 16.5.2014, page 35)
- Commission Regulation (EU) No 601/2014 of 4 June 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food categories of meat and the use of certain food additives in meat preparations (OJ L166, 5.6.2014, page 11)
- Commission Regulation (EU) No 685/2014 of 20 June 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards polyvinyl alcohol-polyethylene glycol-graft-co-polymer in solid food supplements (OJ L182, 21.6.2014, page 23)
- Commission Regulation (EU) No 923/2014 of 25 August 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of aluminium lakes of riboflavins (E 101) and cochineal, carminic acid, carmines (E 120) in certain food categories and Annex to Regulation (EU) No 231/2012 as regards the specifications for riboflavins (E 101) (OJ L252, 26.8.2014, page 11)
- Commission Regulation (EU) No 957/2014 of 10 September 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the removal of montan acid esters (E 912) (OJ L270, 11.9.2014, page 1)
- Commission Regulation (EU) No 969/2014 of 12 September 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Calcium ascorbate (E 302) and Sodium alginate (E 401) in certain unprocessed fruit and vegetables (OJ L272, 13.9.2014, page 8)
- Commission Regulation (EU) No 1084/2014 of 15 October 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of diphosphates (E 450) as a raising agent and acidity regulator in prepared yeast based doughs (OJ L298, 16.10.2014, page 8)
- Commission Regulation (EU) No 1092/2014 of 16 October 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sweeteners in certain fruit or vegetable spreads (OJ L299, 17.10.2014, page 19)
- Commission Regulation (EU) No 1093/2014 of 16 October 2014 amending and correcting Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of certain colours in flavoured ripened cheese (OJ L299, 17.10.2014, page 22)
- Commission Regulation (EU) 2015/537 of 31 March 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of aluminium lakes of cochineal, carminic acid, carmines (E 120) in dietary foods for special medical purposes (OJ L88, 1.4.2015, page 1)
- Commission Regulation (EU) 2015/538 of 31 March 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of benzoic acid — benzoates (E 210-213) in cooked shrimps in brine (OJ L88, 1.4.2015, page 4)
- Commission Regulation (EU) 2015/639 of 23 April 2015 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of silicon dioxide (E 551) in polyvinyl alcohol-polyethylene glycol-graft-co-polymer (E 1209) (OJ L106, 24.4.2015, page 16)
- Commission Regulation (EU) 2015/647 of 24 April 2015 amending and correcting Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of certain food additives (OJ L107, 25.4.2015, page 1)
- Commission Regulation (EU) 2015/649 of 24 April 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of L-leucine as a carrier for table-top sweeteners in tablets (OJ L107, 25.4.2015, page 17)
- Commission Regulation (EU) 2015/1362 of 6 August 2015 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of silicon dioxide (E 551) in extracts of rosemary (E 392) (OJ L210, 7.8.2015, page 22)
- Commission Regulation (EU) 2015/1378 of 11 August 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of riboflavins (E 101) and carotenes (E 160a) in dried potato granules and flakes (OJ L213, 12.8.2015, page 1)
- Commission Regulation (EU) 2015/1739 of 28 September 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of the iron tartrate as an anti-caking agent in salt and its substitutes (OJ L253, 30.9.2015, page 3)
- Commission Regulation (EU) 2015/1832 of 12 October 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Erythritol (E 968) as a flavour enhancer in energy-reduced or with no added sugars flavoured drinks (OJ L266, 13.10.2015, page 27)
- Commission Regulation (EU) 2016/56 of 19 January 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of extracts of rosemary (E 392) in spreadable fats (OJ L13, 20.1.2016, page 46)
- Commission Regulation (EU) 2016/263 of 25 February 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the title of the food category 12.3 Vinegars (OJ L50, 26.2.2016, page 25)
- Commission Regulation (EU) 2016/324 of 7 March 2016 amending and correcting Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of certain food additives permitted in all categories of foods (OJ L61, 8.3.2016, page 1)
- Commission Regulation (EU) 2016/441 of 23 March 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Steviol glycosides (E 960) as a sweetener in mustard (OJ L78, 24.3.2016, page 47)
- Commission Regulation (EU) 2016/479 of 1 April 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of steviol glycosides (E 960) as a sweetener in certain energy-reduced or with no added sugars beverages (OJ L87, 2.4.2016, page 1)
- Commission Regulation (EU) 2016/683 of 2 May 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of propionic acid — propionates (E 280-283) in tortillas (OJ L117, 3.5.2016, page 28)
- Commission Regulation (EU) 2016/691 of 4 May 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of food additives in edible caseinates (OJ L120, 5.5.2016, page 4)
- Commission Regulation (EU) 2016/1776 of 6 October 2016 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sucralose (E 955) as a flavour enhancer in chewing gum with added sugars or polyols (OJ L272, 7.10.2016, page 2)
- Commission Regulation (EU) 2017/335 of 27 February 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of steviol glycosides (E 960) as a sweetener in certain energy-reduced confectionery products (OJ L50, 28.2.2017, page 15)
- Commission Regulation (EU) 2017/839 of 17 May 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of nitrites (E 249-250) in ‘golonka peklowana’ (OJ L125, 18.5.2017, page 7)
- Commission Regulation (EU) 2017/871 of 22 May 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of phosphoric acid — phosphates — di — tri — and polyphosphates (E 338-452) in certain meat preparations (OJ L134, 23.5.2017, page 3)
- Commission Regulation (EU) 2017/874 of 22 May 2017 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of butane (E 943a), isobutane (E 943b) and propane (E 944) in colour preparations (OJ L134, 23.5.2017, page 18)
- Commission Regulation (EU) 2017/1270 of 14 July 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of potassium carbonate (E 501) on peeled, cut and shredded fruit and vegetables (OJ L184, 15.7.2017, page 1)
- Commission Regulation (EU) 2017/1271 of 14 July 2017 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of use of silicon dioxide (E 551) in potassium nitrate (E 252) (OJ L184, 15.7.2017, page 3)
- Commission Regulation (EU) 2017/1399 of 28 July 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards potassium polyaspartate (OJ L199, 29.7.2017, page 8)
- Commission Regulation (EU) 2018/74 of 17 January 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of phosphoric acid — phosphates — di-, tri- and polyphosphates (E 338-452) in frozen vertical meat spits (OJ L13, 18.1.2018, page 21)
- Commission Regulation (EU) 2018/97 of 22 January 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sweeteners in fine bakery wares (OJ L17, 23.1.2017, page 11)
- Commission Regulation (EU) 2018/98 of 22 January 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards calcium sorbate (E 203) (OJ L17, 23.1.2017, page 14)
- Commission Regulation (EU) 2018/677 of 3 May 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Thaumatin (E 957) as a flavour enhancer in certain food categories (OJ L114, 4.5.2018, page 10)
- Commission Regulation (EU) 2018/682 of 4 May 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyglycerol polyricinoleate (E 476) in emulsified sauces (OJ L116, 7.5.2018, page 5)
- Commission Regulation (EU) 2018/1461 of 28 September 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Low-substituted hydroxypropyl cellulose (L-HPC) in food supplements (OJ L245, 1.10.2018, page 1)
- Commission Regulation (EU) 2018/1472 of 28 September 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Cochineal, Carminic acid, Carmines (E 120) (OJ L247, 3.10.2018, page 1)
- Commission Regulation (EU) 2018/1481 of 4 October 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards octyl gallate (E 311) and dodecyl gallate (E 312) (OJ L251, 5.10.2018, page 13)
- Commission Regulation (EU) 2018/1497 of 8 October 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards food category 17 and the use of food additives in food supplements (OJ L293, 9.10.2018, page 36)
- Corrigendum to Commission Regulation (EU) 2018/1497 of 8 October 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards food category 17 and the use of food additives in food supplements (OJ L253, 9.10.2018) (OJ L60, 28.2.2019, page 35)
- Commission Regulation (EU) 2019/800 of 17 May 2019 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the extension of the use of carminic acid, carmine (E 120) in certain meat products traditional in French Overseas Territories (OJ L132, 20.5.2019, page 15)
- Commission Regulation (EU) 2019/801 of 17 May 2019 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of mono- and diglycerides of fatty acids (E 471) on certain fresh fruits (OJ L132, 20.5.2019, page 18)
- Commission Regulation (EU) 2019/891 of 28 May 2019 amending Annexes I and II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the functional class of ‘stabilisers’ and the use of ferrous lactate (E 585) on the mushroom Albatrellus ovinus as a food ingredient in Swedish liver pâtés (OJ L142, 29.5.2019, page 54)
- Commission Regulation (EU) 2019/1676 of 7 October 2019 correcting certain language versions of Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives (OJ L257, 8.10.2019, page 11) [Does not concern the English version]
- Commission Regulation (EU) 2020/268 of 26 February 2020 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sorbic acid (E 200) in liquid colour preparations for the decorative colouring of egg shells (OJ L56, 27.2.2020, page 4)
- Commission Regulation (EU) 2020/279 of 27 February 2020 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of soybean hemicellulose (E 426) (OJ L59, 28.2.2020, page 6)
- Commission Regulation (EU) 2020/771 of 11 June 2020 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Annatto, Bixin, Norbixin (E 160b) (OJ L184, 12.6.2020, page 25)
- Commission Regulation (EU) 2020/1419 of 7 October 2020 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of ascorbic acid (E 300) and citric acid (E 330) on white vegetables intended for further processing (OJ L326, 8.10.2020, page 11)
- Commission Regulation (EU) 2020/1819 of 2 December 2020 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of colours in salmon substitutes (OJ L406, 3.12.2020, page 26)
- Commission Regulation (EU) 2021/1156 of 13 July 2021 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards steviol glycosides (E 960) and rebaudioside M produced via enzyme modification of steviol glycosides from Stevia (OJ L249, 14.12.2021, page 87)
- Commission Regulation (EU) 2021/1175 of 16 July 2021 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyols in certain energy-reduced confectionery products (OJ L256, 19.7.2021, page 53)
- Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171) (OJ L 11, 18.1.2022, page 1)
- Commission Regulation (EU) 2022/141 of 21 January 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of sodium carbonates (E 500) and potassium carbonates (E 501) in unprocessed cephalopods (OJ L 23, 2.2.2022, page 22)
- Commission Regulation (EU) 2022/1023 of 28 June 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of oat lecithin in cocoa and chocolate products as covered by Directive 2000/36/EC of the European Parliament and of the Council (OJ L 172, 29.6.2022, page 5)
- Commission Regulation (EU) 2022/1037 of 29 June 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of glycolipids as a preservative in beverages (OJ L 173, 30.6.2022, page 52)
- Commission Regulation (EU) 2022/1038 of 29 June 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyvinylpyrrolidone (E1201) in food for special medical purposes, in tablet and coated tablet forms (OJ L 173, 30.6.2022, page 56)
- Commission Regulation (EU) 2022/1923 of 10 October 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of ascorbic acid (E 300), sodium ascorbate (E 301) and calcium ascorbate (E 302) in tuna (OJ L 264, 11.10.2022, page 8)
- Commission Regulation (EU) 2023/440 of 28 February 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of carbomer in food supplements (OJ L 64, 1.3.2023, page 4)
- Commission Regulation (EU) 2023/447 of 1 March 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of glucosylated steviol glycosides as sweetener (OJ L 65, 2.3.2023, page 16)
- Commission Regulation (EU) 2023/1329 of 29 June 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyglycerol polyricinoleate (E 476) and the Annex to Commission Regulation (EU) No 231/2012 as regards specifications for glycerol (E 422), polyglycerol esters of fatty acids (E 475) and polyglycerol polyricinoleate (E 476) (OJ L 166, 30.6.2023, page 66)
- Commission Regulation (EU) 2023/2086 of 28 September 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of buffered vinegar as a preservative and acidity regulator (OJ L 241, 29.9.2023, page 73)
- Commission Regulation (EU) 2023/2379 of 29 September 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the food additive stearyl tartrate (E 483) (OJ L, 2023/2379, 03.10.2023)
- Commission Regulation (EU) 2023/2108 of 6 October 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards food additives nitrites (E 249-250) and nitrates (E 251-252) (OJ L, 2023/2108, 09.10.2023)
- Commission Regulation (EU) 2024/346 of 22 January 2024 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of trimagnesium dicitrate in food supplements (OJ L, 2024/346, 23.01.2024)
- Commission Regulation (EU) 2024/374 of 24 January 2024 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the title of the food categories of alcoholic beverages and the use of several additives in certain alcoholic beverages (OJ L, 2024/374, 25.01.2024)
For a pdf copy of the consolidated version of Regulation 1333/2008, see: Regulation 1333/2008 (published July 2023) [Caution - this document is 346 pages long!].
A Commission guidance document is also available:
Specifications of Purity
These have been published in a separate Regulation - Regulation 231/2012 (as amended):
- Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council (OJ L83, 22.3.2012, page 1)
Amendments
- Commission Regulation (EU) No 1050/2012 of 8 November 2012 amending Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards Polyglycitol syrup (OJ L310, 9.11.2012, page 4)
- Commission Regulation (EU) No 25/2013 of 16 January 2013 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the food additive potassium diacetate (OJ L13, 17.1.2013, page 1)
- Commission Regulation (EU) No 497/2013 of 29 May 2013 amending and correcting Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council (OJ L143, 30.5.2013, page 20)
- Commission Regulation (EU) No 724/2013 of 26 July 2013 amending Regulation (EU) No 231/2012 as regards specifications on several polyols (OJ L202, 27.7.2013, page 11)
- Commission Regulation (EU) No 739/2013 of 30 July 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Stigmasterol-rich plant sterols as a stabiliser in ready-to-freeze alcoholic cocktails, and the Annex to Commission Regulation (EU) No 231/2012 as regards specifications for Stigmasterol-rich plant sterols food additive (OJ L204, 31.7.2013, page 35)
- Commission Regulation (EU) No 816/2013 of 28 August 2013 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Neutral methacrylate copolymer and Anionic methacrylate copolymer in solid food supplements and the Annex to Commission Regulation (EU) No 231/2012 as regards the specifications for Basic methacrylate copolymer (E 1205), Neutral methacrylate copolymer and Anionic methacrylate copolymer (OJ L230, 29.8.2013, page 1)
- Commission Regulation (EU) No 817/2013 of 28 August 2013 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Octenyl succinic acid modified gum arabic (OJ L230, 29.8.2013, page 7)
- Commission Regulation (EU) No 1274/2013 of 6 December 2013 amending and correcting Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards certain food additives (OJ L328, 7.12.2013, page 79)
- Commission Regulation (EU) No 264/2014 of 14 March 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyvinylpyrrolidone-vinyl acetate copolymer in solid food supplements and the Annex to Commission Regulation (EU) No 231/2012 as regards its specifications (OJ L76, 15.3.2014, page 22)
- Commission Regulation (EU) No 298/2014 of 21 March 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Magnesium dihydrogen diphosphate for use as raising agent and acidity regulator (OJ L89, 25.3.2014, page 36)
- Commission Regulation (EU) No 497/2014 of 14 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Advantame as a sweetener (OJ L143, 15.5.2014, page 6)
- Commission Regulation (EU) No 506/2014 of 15 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Ethyl lauroyl arginate as a preservative in certain heat-treated meat products (OJ L145, 16.5.2014, page 35)
- Commission Regulation (EU) No 685/2014 of 20 June 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards polyvinyl alcohol-polyethylene glycol-graft-co-polymer in solid food supplements (OJ L182, 21.6.2014, page 23)
- Commission Regulation (EU) No 923/2014 of 25 August 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of aluminium lakes of riboflavins (E 101) and cochineal, carminic acid, carmines (E 120) in certain food categories and Annex to Regulation (EU) No 231/2012 as regards the specifications for riboflavins (E 101) (OJ L252, 26.8.2014, page 11)
- Commission Regulation (EU) No 957/2014 of 10 September 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the removal of montan acid esters (E 912) (OJ L270, 11.9.2014, page 1)
- Commission Regulation (EU) No 966/2014 of 12 September 2014 amending Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for calcium propionate (OJ L272, 13.9.2014, page 1)
- Commission Regulation (EU) 2015/463 of 19 March 2015 amending Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for polyvinyl alcohol (E 1203) (OJ L76, 20.3.2015, page 42)
- Commission Regulation (EU) 2015/649 of 24 April 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of L-leucine as a carrier for table-top sweeteners in tablets (OJ L107, 25.4.2015, page 17)
- Commission Regulation (EU) 2015/1725 of 28 September 2015 amending Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for Ethyl lauroyl arginate (E 243) (OJ L252, 29.9.2015, page 12)
- Commission Regulation (EU) 2015/1739 of 28 September 2015 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of the iron tartrate as an anti-caking agent in salt and its substitutes (OJ L253, 30.9.2015, page 3)
- Commission Regulation (EU) 2016/1814 of 13 October 2016 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for steviol glycosides (E 960) (OJ L278, 14.10.2016, page 37)
- Commission Regulation (EU) 2017/324 of 24 February 2017 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for Basic methacrylate copolymer (E 1205) (OJ L49, 25.2.2017, page 4)
- Commission Regulation (EU) 2017/1399 of 28 July 2017 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards potassium polyaspartate (OJ L199, 29.7.2017, page 8)
- Commission Regulation (EU) 2018/75 of 17 January 2018 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for Microcrystalline cellulose (E460(i)) (OJ L13, 18.1.2018, page 24)
- Commission Regulation (EU) 2018/98 of 22 January 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards calcium sorbate (E 203) (OJ L17, 23.1.2017, page 14)
- Commission Regulation (EU) 2018/681 of 4 May 2018 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for Polyvinyl alcohol-polyethylene glycol graft-co-polymer (E 1209) (OJ L116, 7.5.2018, page 1)
- Commission Regulation (EU) 2018/1461 of 28 September 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Low-substituted hydroxypropyl cellulose (L-HPC) in food supplements (OJ L245, 1.10.2018, page 1)
- Commission Regulation (EU) 2018/1462 of 28 September 2018 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for certain sorbitan esters (E 491 Sorbitan monostearate, E 492 Sorbitan tristearate and E 495 Sorbitan monopalmitate) (OJ L245, 1.10.2018, page 6)
- Commission Regulation (EU) 2018/1472 of 28 September 2018 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards Cochineal, Carminic acid, Carmines (E 120) (OJ L247, 3.10.2018, page 1)
- Commission Regulation (EU) 2018/1481 of 4 October 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards octyl gallate (E 311) and dodecyl gallate (E 312) (OJ L251, 5.10.2018, page 13)
- Commission Regulation (EU) 2020/763 of 9 June 2020 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for tricalcium phosphate (E 341 (iii)) (OJ L182, 10.6.2020, page 8)
- Commission Regulation (EU) 2020/771 of 11 June 2020 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of Annatto, Bixin, Norbixin (E 160b) (OJ L184, 12.6.2020, page 25)
- Commission Regulation (EU) 2021/1156 of 13 July 2021 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards steviol glycosides (E 960) and rebaudioside M produced via enzyme modification of steviol glycosides from Stevia (OJ L249, 14.12.2021, page 87)
- Commission Regulation (EU) 2022/650 of 20 April 2022 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for sodium diacetate (E 262(ii)) (OJ L 119, 21.4.2022, page 65)
- Commission Regulation (EU) 2022/1023 of 28 June 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of oat lecithin in cocoa and chocolate products as covered by Directive 2000/36/EC of the European Parliament and of the Council (OJ L 172, 29.6.2022, page 5)
- Commission Regulation (EU) 2022/1037 of 29 June 2022 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of glycolipids as a preservative in beverages (OJ L 173, 30.6.2022, page 52)
- Commission Regulation (EU) 2022/1396 of 11 August 2022 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the presence of ethylene oxide in food additives (OJ L 211, 12.8.2022, page 182)
- Commission Regulation (EU) 2022/1922 of 10 October 2022 amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for rebaudiosides M, D and AM produced via enzymatic conversion of purified stevia leaf extracts and the specifications for rebaudioside M produced via enzyme modification of steviol glycosides from Stevia (E 960c(i)) (OJ L 264, 11.10.2022, page 1)
- Commission Regulation (EU) 2023/447 of 1 March 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of glucosylated steviol glycosides as sweetener (OJ L 65, 2.3.2023, page 16)
- Commission Regulation (EU) 2023/1329 of 29 June 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of polyglycerol polyricinoleate (E 476) and the Annex to Commission Regulation (EU) No 231/2012 as regards specifications for glycerol (E 422), polyglycerol esters of fatty acids (E 475) and polyglycerol polyricinoleate (E 476) (OJ L 166, 30.6.2023, page 66)
- Commission Regulation (EU) 2023/1428 of 7 July 2023 amending the Annex to Regulation (EU) No 231/2012 as regards mono- and diglycerides of fatty acids (E 471) (OJ L 175, 10.7.2023, page 6)
- Commission Regulation (EU) 2023/2086 of 28 September 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of buffered vinegar as a preservative and acidity regulator (OJ L 241, 29.9.2023, page 73)
- Commission Regulation (EU) 2023/2379 of 29 September 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the food additive stearyl tartrate (E 483) (OJ L, 2023/2379, 03.10.2023)
- Commission Regulation (EU) 2023/2108 of 6 October 2023 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards food additives nitrites (E 249-250) and nitrates (E 251-252) (OJ L, 2023/2108, 09.10.2023)
- Commission Regulation (EU) 2024/346 of 22 January 2024 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of trimagnesium dicitrate in food supplements (OJ L, 2024/346, 23.01.2024)
For a consolideted version of Regulation 231/2012, see: Regulation 231/2012 (published July 2022) [Caution - this document is 288 pages long!]
Procedural Matters
In addition, there have been the following supporting controls:
- Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives (OJ L80, 26.3.2010, page 19)
Amendment:
- Commission Implementing Regulation (EU) 2021/148 of 8 February 2021 amending Regulation (EU) No 257/2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives (OJ L44, 9.2.2021, page 3)
- Commission Regulation (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council establishing a common authorisation procedure for food additives, food enzymes and food flavourings (OJ L64, 11.3.2011, page 15) [For a consolideted version, see: Regulation 234/2011 (published July 2012)]
Amendment:
- Commission Implementing Regulation (EU) No 562/2012 of 27 June 2012 amending Commission Regulation (EU) No 234/2011 with regard to specific data required for risk assessment of food enzymes (OJ L168, 28.6.2012, page 21)
- Commission Implementing Regulation (EU) 2020/1823 of 2 December 2020 amending Regulation (EU) No 234/2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council establishing a common authorisation procedure for food additives, food enzymes and food flavourings (OJ L406, 3.12.2020, page 43)
For more details on the development of these new controls, see the section below: The Development of the new EU Controls
Adding Additives to the List
UK Legislation
Brexit: Prior to the IP Completion Day (31 December 2020), the legal requirements given in the EU Regulations listed above still applied to the UK. Since IP Completion Day, the EU Regulations above have been incorporated into UK legislation but with amendments to correct deficiencies. Information on this is given below. For more details of the process of incorporating EU legislation into UK law, see the separate page: UK Food Law: EU Legislation as Amended for the UK. Provisions for the enforcement of the controls (originally the EU Regulations but now as amended) have been provided in the UK Regulations listed below. For Northern Ireland, EU rules still apply.
Requirements for implementation and enforcement are provided separately for the four parts of the United Kingdom.
1 January 2021: For interim guidance, see:
EU Legislation with links to legislation.gov.uk: amended for application in the UK:
- Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings as amended by:
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Amendment etc.) (EU Exit) Regulations 2019 (SI 2019, No 860) as amended by:
- Food and Feed Hygiene and Safety (Miscellaneous Amendments etc.) (EU Exit) Regulations 2020 (SI 2020 No. 1504)
- Food and Feed (Miscellaneous Amendments) Regulations 2022 (SI 2022, No. 1351)
- Regulation (EC) No. 1333/2008 of the European Parliament and of the Council on food additives as amended by:
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Amendment etc.) (EU Exit) Regulations 2019 (SI 2019, No 860) as amended by:
- Food and Feed Hygiene and Safety (Miscellaneous Amendments etc.) (EU Exit) Regulations 2020 (SI 2020 No. 1504)
- Commission Regulation (EU) No. 231/2012 laying down specifications for food additives listed in Annexes 2 and 3 to Regulation (EC) No. 1333/2008 as amended by:
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Amendment etc.) (EU Exit) Regulations 2019 (SI 2019, No 860) as amended by:
- Food and Feed Hygiene and Safety (Miscellaneous Amendments etc.) (EU Exit) Regulations 2020 (SI 2020 No. 1504)
Enforcement
Requirements for implementation and enforcement are provided separately for the four parts of the United Kingdom.
- England
- Food Additives, Flavourings, Enzymes and Extraction Solvents (England) Regulations 2013 (SI 2013 No 2210)
as amended by:
- Food Information Regulations 2014 (SI 2014, No, 1855)
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Amendment etc.) (EU Exit) Regulations 2019 (2019, No. 860) as amended by:
- Food and Feed Hygiene and Safety (Miscellaneous Amendments etc.) (EU Exit) Regulations 2020 (SI 2020, NO. 1504)
- Food and Feed Safety (Miscellaneous Amendments and Transitional Provisions) Regulations 2022 (SI 2022, No. 377)
- Food Information (Amendment of Transitional Provisions) (England) Regulations 2022 (SI 2022, No. 938)
- Food and Feed (Miscellaneous Amendments) Regulations 2022 (SI 2022, No. 1351)
- Wales
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Wales) Regulations 2013 (SI 2013 No 2591 (W.255)) as amended by:
- Food and Feed Regulated Products (Miscellaneous Amendments) (Wales) (EU Exit) Regulations 2019 (SI 2019, No. 425 (W.99))
- Food (Miscellaneous Amendments) (Wales) (EU Exit) (No. 2) Regulations 2019 (2019, No. 1046 (W.185))
- Food and Feed Hygiene and Safety (Miscellaneous Amendments and Saving Provision) (Wales) (EU Exit) Regulations 2020 (SI 2020, No. 581 (W.331))
- Food, Animal Feed and Seeds (Miscellaneous Amendments and Transitional Provisions) (Wales) (EU Exit) Regulations 2021 (SI 2021, No. 371 (W.114))
- Scotland
- Food Additives, Flavourings, Enzymes and Extraction Solvents (Scotland) Regulations 2013 (SSI 2013 No 266)
as amended by:
- Food Composition, Labelling and Standards (EU Exit) (Scotland) (Amendment) Regulations 2019 (SSI 2019, No. 53) as amended by:
- - Food and Feed (EU Exit) (Scotland) (Amendment) Regulations 2020 (SSI 2020, NO. 372)
- Food Information, Labelling and Standards (EU Exit) (Scotland) (Amendment) Regulations 2019 (SSI 2019, No. 285) as amended by:
- - Food and Feed (EU Exit) (Scotland) (Amendment) Regulations 2020 (SSI 2020, NO. 372)
- Northern Ireland
- Food Additives, Flavourings, Enzymes and Extraction Solvents Regulations (Northern Ireland) 2013 (SRNI 2013 No 220) as originally amended by
- Regulated Products (Amendment) (Northern Ireland) (EU Exit) Regulations (SI 2019, No. 849)[Note: revoked by SI 2020 No. 1504]
Guidance Document
This page was first provided on 29 July 1996
To go to main Foodlaw-Reading
Index page, click here.
![]() Please complete a quick survey on a Google Form - LINK
Please complete a quick survey on a Google Form - LINK


